
Maxence Duprez
Cofondateur de CIKLab
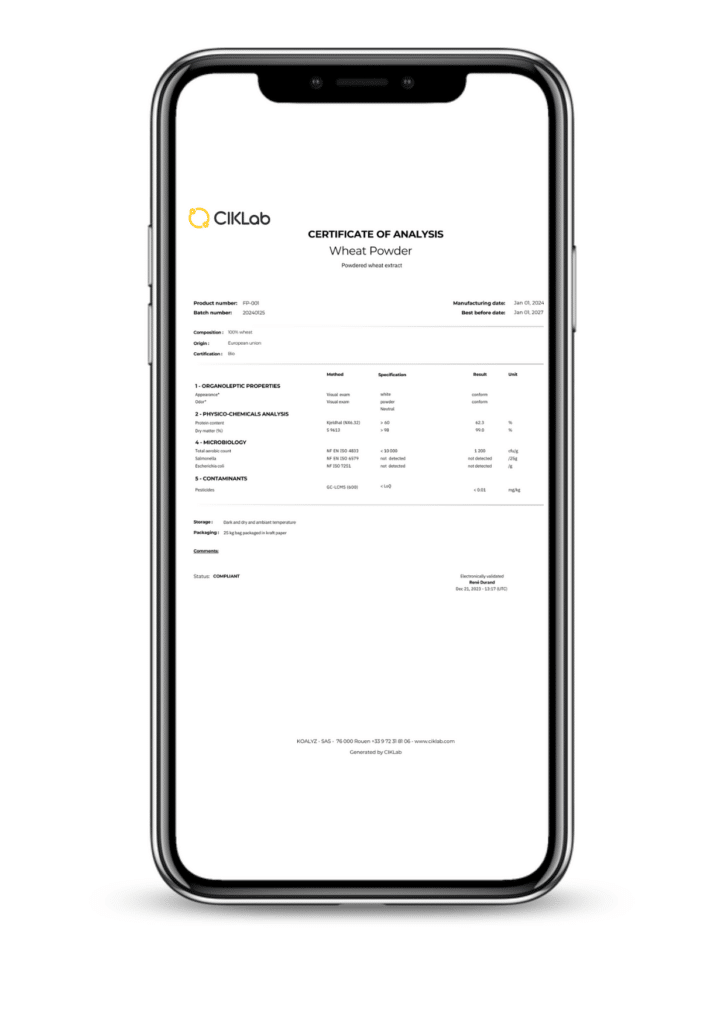
Paper sellers…? In some industries, such as ingredients, “quality documents” are more important than the product itself. Surprising? Perhaps, but it’s hardly an exaggeration.
What documents fall under this term?
All customers, regardless of their position in the value chain, scrutinize the data in their suppliers’ certificates of analysis. Sometimes, it is the sole check performed to release a product.
Documentation is a symbol of seriousness and trust. It is the technical documentation, more than a commercial brochure, that enables your customers to choose a product.
A comprehensive and well-made technical sheet is the initial proof of your product’s quality.
If the quality documentation is lacking, how can one expect the product to be otherwise?
Hence, offering impeccable quality documentation, both in substance and form, is essential. In addition to being a technical document, it is the first marketing and commercial argument.
From a Quality perspective, a product is merely a structured combination of criteria, specifications, commitments, analyses, and results.
A Certificate of Analysis corresponds to a specification sheet or technical document.
The technical document outlines the theoretical parameters that a product must adhere to. The Certificate of Analysis provides precise results specific to a batch.
Ideally, the Certificate of Analysis exactly reflects the information in the specification sheet, adding details about the specific batch and the test results. It is crucial to ensure that Certificates of Analysis and specification sheets are updated simultaneously.
Regulations vary across industries regarding the information required on specification sheets and Certificates of Analysis, but generally, similar information is included.
| Specification Sheet | CoA |
|
|
There is sometimes a bit of confusion between certificates of analysis and certificates of conformity.
Certainly, each industry has its standards and practices, but in theory:
For ingredients, most certificates typically include both analysis results and compliance information.
Generally, physico-chemical parameters, active ingredients, and microbiological activity are systematically tested. However, contaminants (mycotoxins, heavy metals, pesticides, PAHs) are tested periodically.
Most manufacturers do not have dedicated quality control software to automate the generation of Certificates of Analysis (CoAs). The entire quality control process is managed using Excel files and Word documents.
As a result, generating Certificates of Analysis is a very tedious task for quality managers who must compile data, enter information, verify, record, and validate these documents.
The simplest way to proceed is to create a standard template certificate for each product or product family. For each batch, you only need to modify the necessary information (lot number, expiration date, validation date, etc.) and update the analysis results.
Once the certificate of analysis is ready, it needs to be printed as a .pdf, saved in the appropriate folder following the naming convention for easy retrieval, and then validated by adding a signature.
Since everything is manual, it’s possible to generate customized certificates based on customer requirements.
However, filling out the certificates of analysis requires a lot of attention to avoid data entry errors. Additionally, it is a very time-consuming activity, taking an average of 10 to 15 minutes per certificate.
For a company releasing 200 batches per year, this represents 50 hours, equivalent to 1500€.
Without extensive knowledge of Excel and Word, using simple lookup formulas can partially automate the process. You can generate a Certificate of Analysis from information entered into an Excel database. This will save time in filling out the document and, most importantly, help avoid input errors or copy/paste mistakes.
Steps:
Step 1: Begin by grouping your products that share the same analyses.
In a first tab, create your database of analysis results.
| Produit | Lot | ANALYSE 1 | RESUL. 1 | ANALYSE 2 | RESUL. 2 | Etc. |
| Produit A | 1234 | >10% | 11% | Conform | Conform | |
| Produit B | 6789 | >5% | 6% | Conform | Conform |
Step 2: Define print templates and retrieve information based on the lot number, for example.
| Produit A | |||
| N° Lot | 1234 | ||
| Spec | Result | ||
| Analyse 1 | >10% | 11% | |
| Analyse 2 | Conform | Conform |
| Produit B | |||
| N° Lot | 6789 | ||
| Spec | Result | ||
| Analyse 1 | >5% | 6% | |
| Analyse 2 | Conform | Conform |
This will save you a lot of time, provided you have a limited number of products or families. Unfortunately, files become heavy quickly, and queries take too long, especially when dealing with numerous products. Moreover, adapting certificate templates to client or product specifics becomes more challenging.
Similarly, updating quality documents can be a real headache. In fact, each change must be made in duplicate, on the technical sheet and on the certificate of analysis.
⚠️ In an automated system with formulas, each change can break the formulas.
In a manual system, you may have to modify each individual file:
With CIKLab, the quality control management software, generating Certificates of Analysis (CoA) is fully automated.
The CoAs mirror the specification sheets, creating a dynamic system where the specification sheets and CoAs are interconnected. Any changes made to the specification sheet automatically update the Certificate of Analysis as needed.
In CIKLab, you begin by registering and centralizing your products and associating specifications, commitments, and required analyses.
From your catalog:
If needed, you can customize the design to match your visual identity.
If you manage different specifications for your suppliers or if a client has specific requirements, you can easily handle it in CIKLab.
When you create a new batch, CIKLab automates the required analyses based on your control plan and orders them from your subcontracted laboratories.
Upon receiving the reports, the analysis results are entered into CIKLab, and a validation process allows for closing and releasing the batches.
>> Your Certificates of Analysis are generated with a single click.
They are directly signed and saved as PDFs, named according to a precise nomenclature, and directly linked to your products and batches. All documentation is available in your online library. You no longer waste time organizing your documents in a complex folder structure. You can even grant your sales representatives access to technical sheets and Certificates of Analysis while restricting access to other quality documents.
To generate a customer-specific technical sheet or Certificate of Analysis, it’s even faster.
Starting with your standard product, you create the customer’s specifications and generate their technical sheet. To produce a Certificate of Analysis, you can import the results from your standard product and generate the certificate that corresponds to the customer’s specifications.
Traceability management allows you to trace back to the source product without the risk of errors.
With CIKLab, you can easily generate your Certificates of Analysis, minimize the risk of errors, and save valuable time in quality control management.

Cofondateur de CIKLab
Koalyz SAS
107, allée François Mitterrand
76100 ROUEN – France